Refer to the figures below. 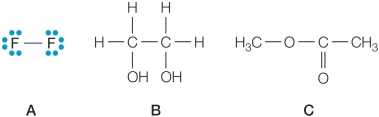 Which compound would have a higher heat of vaporization than water, and why?
Which compound would have a higher heat of vaporization than water, and why?
A) Compound A because it is smaller in size than water.
B) Compound A because unlike water, it is not capable of hydrogen bonding.
C) Compound B because it can form more hydrogen bonds per molecule than water.
D) Compound B because it contains more covalent bonds per molecule than water.
E) Compound C because it contains more oxygen atoms per molecule than water.
Correct Answer:
Verified
Q65: Which characteristic of water contributes most to
Q66: Which statement explains why ice floats in
Q67: Refer to the balanced chemical equation
Q68: What features of the water molecule are
Q69: Refer to the reaction shown. C3H8
Q71: Phosphate ion has the structure PO43−.This ion
Q72: In the summer, ice is used to
Q73: Which observation makes a strong case that
Q74: A mole of hydrogen and a mole
Q75: How would you make 100 mL of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents