Refer to the figure below. 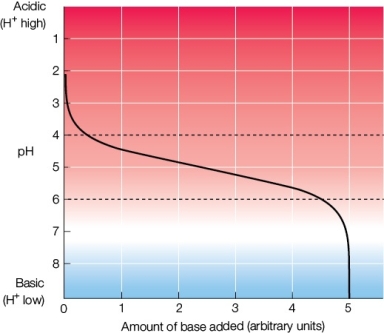 Which part of the diagram has the largest H+ change per unit of base added?
Which part of the diagram has the largest H+ change per unit of base added?
A) The area between the dotted lines
B) The area between pH 2 and pH 4
C) The area between pH 4 and pH 6
D) The area between pH 6 and pH 8
E) The areas between pH 2 and pH 4 and between pH 6 and pH 8
Correct Answer:
Verified
Q86: Every atom except _ has one or
Q87: The chemical properties of an element are
Q88: Of the different types of chemical bonds,
Q89: Oxygen and carbon are defined as different
Q90: A _ links two or more atoms
Q92: The mass of a proton serves as
Q93: An atom has 36 protons and 44
Q94: A solution with pH 9 contains
A) more
Q95: H2SO4 can ionize completely to yield two
Q96: When 0.1 mole of sodium hydroxide (NaOH)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents