A molecule contains five atoms and has a molecular weight of 85 g per mole.The atoms are of elements with atomic numbers 1, 6, and 17.Which molecular structure could represent this molecule?
A) 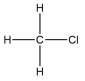
B) 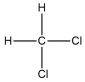
C) 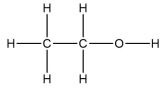
D) 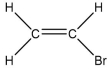
E) 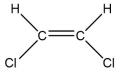
Correct Answer:
Verified
Q233: The reactivity of an atom arises from
Q234: Which finding can be used to justify
Q235: Which interaction between atoms is the strongest?
A)
Q236: Polar molecules
A) have electric charges that are
Q237: Isotopic analysis of biological samples can be
Q239: Covalent bond formation depends on the ability
Q240: Refer to the table below. 
Q241: Of the statements below, which best explains
Q242: Which property of water contributes most to
Q243: Why is the pH of a 0.1
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents