Refer to the table below. 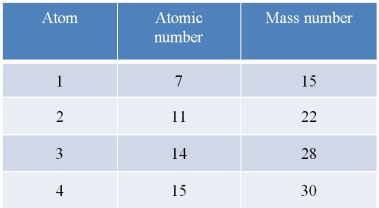 Which statement about the atoms in the table is accurate?
Which statement about the atoms in the table is accurate?
A) Atom 1 and atom 2 are isotopes of the same element.
B) Atom 1 and atom 4 have the same number of electrons in their outer shells.
C) Atom 3 and atom 4 have the same number of neutrons in their nuclei.
D) Atom 2 and atom 3 differ by six neutrons in their nuclei.
E) Atom 1 and atom 4 gain stability when they each lose one electron.
Correct Answer:
Verified
Q235: Which interaction between atoms is the strongest?
A)
Q236: Polar molecules
A) have electric charges that are
Q237: Isotopic analysis of biological samples can be
Q238: A molecule contains five atoms and has
Q239: Covalent bond formation depends on the ability
Q241: Of the statements below, which best explains
Q242: Which property of water contributes most to
Q243: Why is the pH of a 0.1
Q244: Given that Avogadro's number is 6.02 ×
Q245: Sweating is a useful cooling device for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents