Refer to the figure below showing the change in free energy resulting from a chemical reaction. 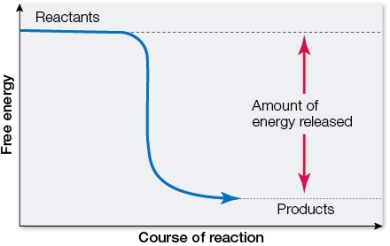 Which statement about the reaction is true?
Which statement about the reaction is true?
A) It is an endergonic reaction.
B) The reactants have less energy than the products.
C) G is negative.
D) It is an example of a condensation reaction.
E) It is an anabolic reaction.
Correct Answer:
Verified
Q9: The sum total of all chemical reactions
Q10: Which of the following represents kinetic energy?
A)
Q11: During photosynthesis, plants use light energy to
Q12: Free energy is
A) enthalpy.
B) entropy.
C) usable energy.
D)
Q13: Refer to the table below. 
Q15: The
Q16: When energy is converted from one form
Q17: Chemical equilibrium
A) is a static state
Q18: How does the second law of thermodynamics
Q19: A plant makes sugars via photosynthesis
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents