Refer to the table below. 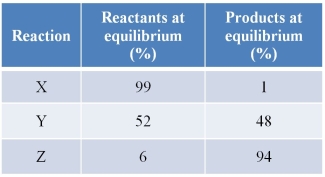 How do you predict the G° values for these reactions will compare?
How do you predict the G° values for these reactions will compare?
A) All of the reactions will have positive G° values, with Z lowest and X highest.
B) All of the reactions will have negative G° values, with Z having the least negative and X the greatest negative value.
C) Y will have the smallest G° value, while X will have a large positive G° and Z will have a large negative G°.
D) Y will have the smallest G° value, while X will have a large negative G° and Z will have a large positive G°.
E) Y will have the largest G° value, while X will have a small positive G° and Z will have a small negative G°.
Correct Answer:
Verified
Q144: Where is the chemical energy that is
Q145: In order to measure the standard
Q146: Which action could indicate whether a given
Q147: Which type of reaction is used to
Q148: Refer to the figure below. 
Q150: The creation of ATP from ADP requires
Q151: The energy released by the hydrolysis
Q152: In a biological system, you want to
Q153: Which of the following could we
Q154: In solution, ATP is readily hydrolyzed into
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents