Refer to the figure below, which shows an oxidation-reduction reaction that occurs spontaneously. 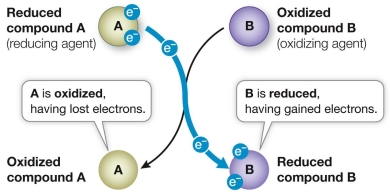 Which statement correctly compares the free energy stored in molecules shown in the figure?
Which statement correctly compares the free energy stored in molecules shown in the figure?
A) The reduced form of B has greater free energy than the reduced form of A.
B) The oxidized form of B has greater free energy than the reduced form of A.
C) The reduced form of A has greater free energy than the oxidized form of A.
D) The oxidized form of B has greater free energy than the reduced form of B.
E) The oxidized form of A has greater free energy than the reduced form of B.
Correct Answer:
Verified
Q4: The carbon end product of glycolysis is
A)
Q5: The oxidation of malate to oxaloacetate is
Q6: Refer to the figure below, which shows
Q7: Which statement about metabolic pathways is false?
A)
Q8: NADH
A) is a key electron carrier in
Q10: Glycolysis converts glucose to pyruvate with the
Q11: Refer to the figure below, illustrating how
Q12: Refer to the figure below. 
Q13: Refer to the reaction below.
Glyceraldehyde
Q14: Refer to the figure below, showing the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents