Refer to the table below. 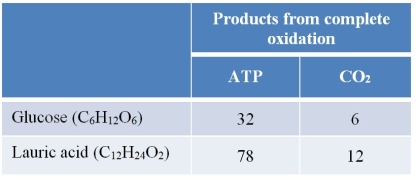 The table summarizes the products (in moles) of the complete oxidation of 1 mole of glucose and the complete oxidation of 1 mole of lauric acid, a fatty acid.Use this information to determine how energy production will compare when an equivalent amount of carbon dioxide is produced from oxidation events involving these two molecules.
The table summarizes the products (in moles) of the complete oxidation of 1 mole of glucose and the complete oxidation of 1 mole of lauric acid, a fatty acid.Use this information to determine how energy production will compare when an equivalent amount of carbon dioxide is produced from oxidation events involving these two molecules.
A) The energy production will be equal for equal quantities of CO2 production by these two molecules.
B) The energy production by lauric acid oxidation will be greater by 10 ATP when the two molecules are fully oxidized to produce equal quantities of CO2.
C) The energy production by glucose oxidation will be greater by 10 ATP when the two molecules are fully oxidized to produce equal quantities of CO2.
D) The energy production by lauric acid oxidation will be greater by 14 ATP when the two molecules are fully oxidized to produce equal quantities of CO2.
E) The energy production by glucose oxidation will be greater by 14 ATP when the two molecules are fully oxidized to produce equal quantities of CO2.
Correct Answer:
Verified
Q56: According to the chemiosmotic theory, the energy
Q57: During cellular respiration, H2O is produced as
Q58: Increasing the body's brown fat could be
Q59: In the absence of O2, cells capable
Q60: The oxidizing agent at the end of
Q62: Key events in the rise of multicellularity
Q63: Bacteria that are shifted from an oxygen-rich
Q64: Refer to the table below. 
Q65: Most ATP produced in aerobic organisms is
Q66: The fermentation process in vertebrate muscle cells
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents