Refer to the graphs below, illustrating the free energy changes that occur during reactions in which glucose is oxidized to carbon dioxide and water. 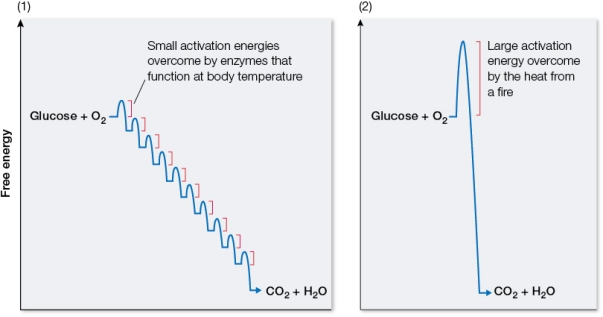 Identify which graph corresponds to the laboratory oxidation of glucose and which corresponds to cellular oxidation of glucose.Explain how the overall free energy change compares in these two reactions and why.Also compare the form that the released energy takes in these two reactions.
Identify which graph corresponds to the laboratory oxidation of glucose and which corresponds to cellular oxidation of glucose.Explain how the overall free energy change compares in these two reactions and why.Also compare the form that the released energy takes in these two reactions.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q112: Refer to the diagram below, showing energy-releasing
Q113: A research scientist divides mouse liver cells
Q114: The formation of glucose from glycolytic and
Q115: Refer to the graph below. 
Q116: The chemiosmotic formation of ATP during the
Q118: The compound citrate is generated as part
Q119: The oxidation of acetyl CoA to CO2
Q120: Workers in wine production facilities have an
Q121: Refer to the diagram below, showing energy-releasing
Q122: In eukaryotes, the organelle containing the enzyme(s)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents