Refer to the figure below. 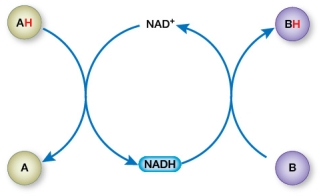 Which half-reactions represent a change from more stored free energy to less stored free energy?
Which half-reactions represent a change from more stored free energy to less stored free energy?
A) B BH and NAD+ NADH
B) AH A and B BH
C) AH A and NAD+ NADH
D) AH A and NADH NAD+
E) B BH and NADH NAD+
Correct Answer:
Verified
Q139: The rise of oxygen in Earth's atmosphere
Q140: Metabolic pathways are
A) small sets of chemical
Q141: How are steps 1-3 of glycolysis different
Q142: What is the cellular location of glycolysis
Q143: Refer to the figure below. 
Q145: A biochemist discovers several new microorganisms in
Q146: Which list places the molecules, from left
Q147: Which reaction is not an example
Q148: Which statement regarding glycolysis is true?
A) Two
Q149: Refer to the figure below. 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents