The stability of 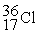 with respect to alpha,beta-plus,and beta-minus decay is to be determined.The following atomic masses in amu are known:
with respect to alpha,beta-plus,and beta-minus decay is to be determined.The following atomic masses in amu are known: 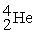 4.002603
4.002603 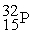 31.973907
31.973907 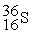 35.967081
35.967081 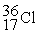 35.968307
35.968307 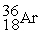 35.967546 The
35.967546 The 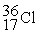 nuclide is
nuclide is
A) not subject to alpha,beta-plus,or beta-minus decay.
B) subject to alpha decay only.
C) subject to beta-plus decay only.
D) subject to beta-minus decay only.
E) subject to beta-plus or beta-minus decay,but not to alpha decay.
Correct Answer:
Verified
Q37: Because neutrons and protons are approximately 1837
Q38: In massive stars,three helium atoms fuse together,forming
Q39: Nuclei that are all isotopes of an
Q40: If half of a radioactive substance decays
Q41: Which of the following is not true
Q43: The half-lives of cobalt-60 and strontium-90 are
Q44: Suppose the half-life of some element is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents