A small piece of aluminum (atomic number 13) contains 1015 atoms.(The atomic number is the number of protons;it determines the (positive) electric charge of the nucleus and,thus,the number of electrons in a neutral atom. ) If the piece of aluminum has a net positive charge of 3.0 μC,what fraction of the electrons that the aluminum had when it was neutral would have had to be lost?
A) 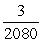
B) 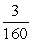
C) 1.9 × 10-20
D) 1.4 × 10-21
Correct Answer:
Verified
Q8: Two like charges of the same magnitude
Q9: A 3.0 μC positive charge is located
Q10: A small glass bead has been charged
Q11: Two equally charged spheres of mass 1.0
Q12: The electric field 2.8 cm from a
Q14: What is the electric field strength at
Q15: In the figure Q = 5.8 nC
Q16: In the figure below the charge in
Q17: Suppose a van de Graaff generator builds
Q18: A proton is located at x =
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents