A heat engine takes 2.0 moles of an ideal gas through the reversible cycle abca, on the pV diagram shown in the figure. The path bc is an isothermal process. The temperature at c is 820 K, and the volumes at a and c are 0.010 m3 and 0.16 m3, respectively. The molar heat capacity at constant volume, of the gas, is 37 J/mol·K, and the ideal gas constant is R = 8.314 J/(mol∙K) . The thermal efficiency of the engine is closest to 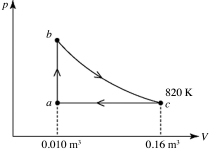
A) 0.26.
B) 0.026.
C) 0.33.
D) 0.40.
E) 0.53.
Correct Answer:
Verified
Q4: An engine manufacturer makes the claim that
Q15: The entropy of an isolated system must
Q15: A heat engine performs the reversible cycle
Q16: An automobile engine takes in 4000 J
Q18: A refrigerator has a coefficient of performance
Q20: A real (non-Carnot) heat engine, operating between
Q22: A Carnot refrigerator takes heat from water
Q23: One of the most efficient engines built
Q23: A certain Carnot heat pump transfers energy
Q32: A Carnot refrigerator has a coefficient of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents