What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol∙K) . 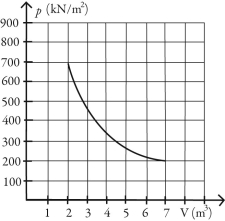
A) 221 J/K
B) 104 J/K
C) 63.1 J/K
D) 45.2 J/K
E) 90.8 J/K
Correct Answer:
Verified
Q40: A Carnot engine operates between reservoirs at
Q41: A 810-g quantity of ethanol, in the
Q42: A 610-g quantity of an ideal gas
Q43: At atmospheric pressure, 45 moles of liquid
Q44: A brass rod, 75.0 cm long and
Q44: A system consists of two very large
Q45: A 2.0-kg block of aluminum at 50°C
Q45: A 2.00 kg piece of lead at
Q47: A 2.00 kg piece of lead at
Q50: A 2.00-kg block of ice at 0.00°C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents