An electron inside a hydrogen atom is confined to within a space of 0.110 nm. What is the minimum uncertainty in the electron's velocity? ( 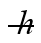 = 1.055 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg)
= 1.055 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg)
A) 5.26 × 105 m/s
B) 7.50 × 105 m/s
C) 5.26 × 107 m/s
D) 7.50 × 107 m/s
E) 5.26 × 109 m/s
Correct Answer:
Verified
Q28: A laser produces a beam of 4000-nm
Q29: A molecule of roughly spherical shape has
Q30: A photon of wavelength 18.0 pm is
Q31: The energy of an electron state has
Q32: The excited state of a certain atom
Q33: A nonrelativistic electron is confined to a
Q35: A 440-nm spectral line is produced by
Q36: A beam of x-rays at a certain
Q37: A photon of wavelength 29 pm is
Q38: A nonrelativistic proton is confined to a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents