The atoms in a nickel crystal vibrate as harmonic oscillators with an angular frequency of 2.3 × 1013 rad/s. The mass of a nickel atom is 9.75 × 10-26 kg. What is the difference in energy between adjacent vibrational energy levels of nickel? (h = 6.626 × 10-34 J ∙ s, 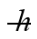 = 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 0.015 eV
B) 0.019 eV
C) 0.023 eV
D) 0.027 eV
E) 0.031 eV
Correct Answer:
Verified
Q26: The lowest energy level of a certain
Q30: An electron is trapped in an infinite
Q32: You want to have an electron in
Q33: Calculate the ground state energy of a
Q34: A 3.10-eV electron is incident on a
Q35: A 10.0-g bouncy ball is confined in
Q36: An electron is confined in a one-dimensional
Q37: An electron is confined in a harmonic
Q39: Find the wavelength of the photon emitted
Q40: One fairly crude method of determining the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents