Uranium-238 decays into thorium-234 plus an alpha particle. How much energy is released in this process? 1 u = 931.494 MeV/c2, and the relevant mass values are 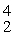 He: 4.002603 u
He: 4.002603 u 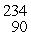
Th: 234.043583 u 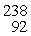
U: 238.050786 u
A) 4.28 MeV
B) 3.76 MeV
C) 3.18 MeV
D) 2.89 MeV
E) 5.05 MeV
Correct Answer:
Verified
Q29: Two identical nuclei of mass 18 u
Q32: How does the mass of the products
Q33: What is the binding energy per nucleon
Q35: If a nucleus had a diameter of
Q36: The following masses are known: 
Q38: Plutonium-239 decays into uranium-235 plus an alpha
Q39: The carbon in your body was formed
Q40: The neutral deuterium atom, Q41: What mass of 14C (having a half-life Q67: Carbon-14 has a half-life of 5730 y.A![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents