The stability of 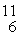 C with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
C with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 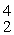
He: 4.002603 u 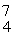
Be: 7.016928 u 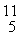
B: 11.009305 u 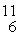
C: 11.011433 u 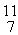
N: 11.026742 u
The 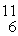
C nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+ decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
Correct Answer:
Verified
Q53: Rutherfordium-261 has a half-life of 1.08 min.How
Q54: An isotope of Tc having a half-life
Q55: Scandium, Q57: A sphere made of a radioactive isotope Q58: In a laboratory accident a work area Q60: A hospital patient has been given some Q61: In the nuclear reaction![]()
n + 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents