Consider the fusion reaction 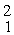
H + 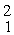
H + 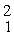
H → 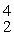
He + 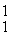
H + 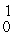
n
The atomic masses are: 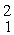
H: 2.01410 u 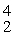
He: 4.00260 u 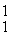
H, 1.00783 u 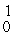
n: 1.008665 u
What mass of deuterium fuel, 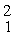
H, is used up in producing 8.2 × 1013 J of energy by this reaction? (1u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
Correct Answer:
Verified
Q79: How much energy is released in the
Q80: An ancient rock is found to contain
Q82: A 70-kg laboratory technician absorbs 2.9 mJ
Q83: A certain isotope has a half-life of
Q83: The reaction 2H + 2H →? 3H
Q84: The radioactive nuclei 60Co is widely used
Q85: Two deuterium nuclei, Q86: A 57-kg researcher absorbs 6.3 × 108 Q89: The maximum permissible workday dose for occupational Q89: Two deuterium nuclei, ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents