A gas expands as shown in the graph.If the heat taken in during this process is 1.02 * 106 J and 1 atm = 1.01 * 105 N/m2,the change in internal energy of the gas (in J) is 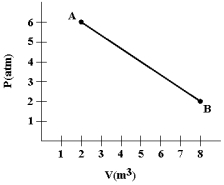
A) -2.42 *106
B) -1.40 * 106
C) -1.02 * 106
D) 1.02 * 106
E) 1.40 * 106
Correct Answer:
Verified
Q7: In an adiabatic free expansion
A) no heat
Q15: The internal energy of n moles of
Q27: Which statement below regarding the First Law
Q30: A gas expands from A to B
Q33: The work done in the expansion from
Q34: Determine the work done by 5
Q34: In an isobaric process
A) the volume remains
Q38: A 100-g cube of ice is
Q40: Gas in a container increases its pressure
Q51: Which of the following statements is(are) correct
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents