Which of the following is the Lewis structure for CH3CH2O2H?
A) 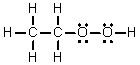
B) 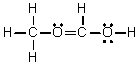
C) 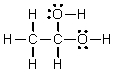
D) 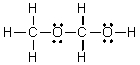
E) 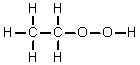
Correct Answer:
Verified
Q1: The greatest degree of ionic character is
Q2: Considering Lewis structures,which of these compounds possesses
Q9: Which of the following is the Lewis
Q10: Consider the following: CH3CH2CH=CHCH2CH3
CH3CH2CH2CH2CH=CH2
I
II
CH3CH=CHCH2CH2CH3
CH2=CHCH2CH2CH2CH3
III
IV
Which two structures represent
Q11: Which of the following is a set
Q12: Which compound is not a constitutional isomer
Q13: CH3CH2OCH2CH3 and CH3CH2CH2CH2OH are examples of what
Q16: Which of these is a correct electron-dot
Q17: Which is NOT a correct Lewis structure?
A)
Q19: Which compound is not a constitutional isomer
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents