In which structure(s) below does the oxygen have a formal charge of +1? 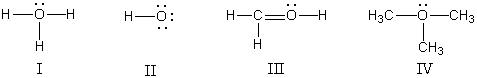 s
s
A) I only
B) II only
C) I and III
D) I and IV
E) I,III,and IV
Correct Answer:
Verified
Q1: The greatest degree of ionic character is
Q17: Which is NOT a correct Lewis structure?
A)
Q19: Which compound is not a constitutional isomer
Q20: Which of the following represent a pair
Q21: Which of the following species is/are a
Q23: What is the formal charge on carbon
Q24: Which of the following species is/are not
Q25: Which compound contains a nitrogen atom with
Q26: What is the formal charge on oxygen
Q27: Expansion of the valence shell to accommodate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents