Listed below are electron dot formulas for several simple molecules and ions.All valence electrons are shown;however,electrical charges have been omitted deliberately. 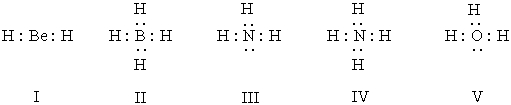 Which of the structures actually bear(s) a positive charge?
Which of the structures actually bear(s) a positive charge?
A) I
B) II
C) III
D) III & V
E) IV & V
Correct Answer:
Verified
Q25: Which compound contains a nitrogen atom with
Q26: What is the formal charge on oxygen
Q27: Expansion of the valence shell to accommodate
Q28: In which structure(s)below does nitrogen have a
Q29: Which of the following molecules or ions
Q31: The formal charge on sulfur in sulfuric
Q32: Listed below are electron dot formulas for
Q33: What is the formal charge on oxygen
Q34: Which of the following is an ion
Q35: Which of the following pairs are NOT
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents