Consider the following: 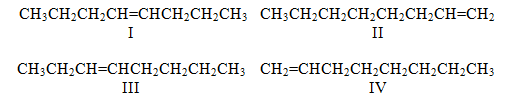
Which structures can exist as cis-trans : isomers?
A) I and II
B) I and III
C) I and IV
D) II and III
E) I alone
Correct Answer:
Verified
Q74: Which of the following species contributes more
Q75: Identify the atomic orbital.The lone pair electrons
Q76: According to molecular orbital theory,in the
Q76: What is the geometry of the C
Q77: The C4-C5 carbon-carbon bond in the following
Q80: Identify the atomic orbitals in the C-H
Q81: What is the hybridization of the O
Q83: Identify the atomic orbitals in the C-N
Q101: The bond angles for the bold-faced
Q116: Which molecule has a non-linear shape
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents