Identify the atomic orbitals in the C-N sigma bond in the following oxime: 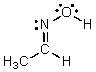
A) (2sp2,2sp2)
B) (2sp3,2sp3)
C) (2sp,2sp)
D) (2sp2,2sp3)
E) (2sp,1s)
Correct Answer:
Verified
Q76: According to molecular orbital theory,in the
Q79: Consider the following: Q80: Identify the atomic orbitals in the C-H Q81: What is the hybridization of the O Q85: What is the hybridization of the N Q86: Based on VSEPR theory,which of the following Q87: In which molecule is the central atom Q88: Identify the atomic orbital.The lone pair electrons Q101: The bond angles for the bold-faced Q116: Which molecule has a non-linear shape![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents