Identify the atomic orbital.The lone pair electrons on the N atom are contained in: 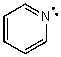
A) 2sp2
B) 2sp3
C) 2p
D) 2s
E) 2sp
Correct Answer:
Verified
Q18: Which of these substances contain both covalent
Q109: The bond angle for the C-C-O
Q110: Based on the VSEPR theory,which of the
Q111: Which of these structures would be a
Q112: What is the geometry of the N
Q113: Identify the atomic orbital.The lone pair electrons
Q115: In which molecule(s)can the molecular geometry be
Q118: The bond angle for the C-S-C
Q124: Which molecule would be linear? (In each
Q134: The bond angle for the C-C-N
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents