The following electron configuration represents _______ 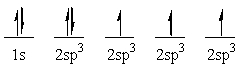
A) the ground state of boron.
B) the sp3 hybridized state of carbon.
C) the sp3 hybridized state of nitrogen.
D) the ground state of carbon.
E) an excited state of carbon.
Correct Answer:
Verified
Q125: The following electron configuration represents: 
Q128: Different compounds with the same molecular formula
Q133: Which molecule contains an sp-hybridized carbon?
A) HCN
B)
Q134: Identify the atomic orbitals involved in the
Q141: Draw all isomers of C4H8,using bond-line formulas.
Q143: Draw all the isomers of C4H10O,using bond-line
Q152: The modern definition of organic chemistry is
Q153: Constitutional isomers differ in the _.
Q155: The bond that results when two atoms
Q156: Define an orbital.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents