Which of these would you expect to have the lowest boiling point?
A) CH3CH2CH2OH
B) 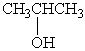
C) CH3OCH2CH3
D) CH3CH2CH2CH2OH
E) CH3CH2OCH2CH3
Correct Answer:
Verified
Q64: Which compound would have the lowest boiling
Q66: Of the following compounds,the one with the
Q67: What functional group(s)is/are present in the following
Q68: Which compound would have the highest boiling
Q69: Which alkane is predicted to have the
Q71: Drawn below is Atropine,found in Atropa belladonna,sometimes
Q73: What intermolecular forces hold base pairs together
Q73: The compound shown below is a substance
Q76: Which compound would you expect to have
Q77: Which compound would you expect to have
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents