Which SN2 reaction would you expect to take place most rapidly? Assume that the concentrations of the reactants and the temperature are the same in each instance:
A) 
B) 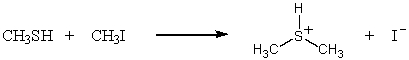
C) 
D) 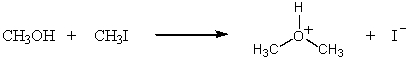
E) 
Correct Answer:
Verified
Q24: An increase in the kinetic energy of
Q26: Ambident nucleophiles are ones which can react
Q27: By analyzing the starting material and the
Q29: Which alkyl chloride,though primary,is essentially unreactive in
Q30: Which is a true statement concerning the
Q33: Which of the following reactions proceeds with
Q34: The Hammond-Leffler postulate,when applied to nucleophilic substitutions
Q36: By analyzing the starting material and the
Q136: Consider the substitution reaction that takes place
Q147: You want to synthesize 3-methyl-2-pentene from 2-chloro-3-methylpentane.Which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents