The broadband proton-decoupled 13C NMR spectrum of a hexyl chloride exhibits five signals.Which of these structures could be the correct one for the compound?
A) 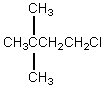
B) 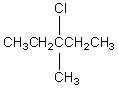
C) 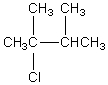
D) 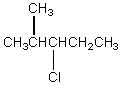
E) 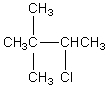
Correct Answer:
Verified
Q64: How will the methyl carbon appear in
Q65: Determine the likely structure for a
Q66: How many 13C signals would 1,4-dimethylbenzene give?
Q67: A prominent (M 1+
Q68: How many 13C signals would 1,3-dichlorobenzene give?
Q70: How many signals will be recorded in
Q71: Select the structure of a compound C6H14
Q72: A compound with the molecular formula
Q74: What is the structure of the compound
Q95: The C7 compound which gives 3 signals
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents