Which of the following reactions would have the smallest energy of activation?
A) CH4 + Br· CH3· + HBr
B) CH3CH3 + Br· CH3CH2· + HBr
C) 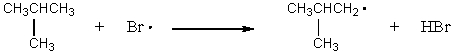
D) 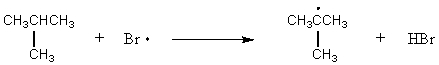
E) 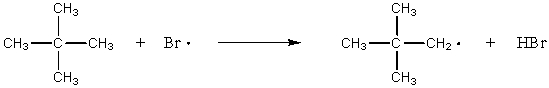
Correct Answer:
Verified
Q16: At some temperatures,the relative reactivities of 3°,2°
Q28: What feature would you expect to see
Q30: Which reaction would you expect to
Q31: An example of a reaction having
Q32: What sequence of reactions could be
Q34: An example of a reaction having
Q35: Which reaction would you expect to
Q36: Mono-bromination of the following alkane, 
Q37: Which of the following reactions should
Q38: When an alkane in which all hydrogen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents