Which carbon of 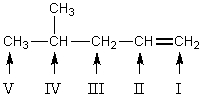 is predicted to be the major site of substitution when this alkene reacts with chlorine at 400 C?
is predicted to be the major site of substitution when this alkene reacts with chlorine at 400 C?
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q5: The allyl radical has how many
Q7: Which hydrogen atom(s)of Q11: What product(s)would you expect from the following Q14: What product(s)would you expect from the following Q15: The LUMO of the allylic radical has Q15: Which free radical would be most stable? Q16: The HOMO of the allylic radical has Q17: Which would be the best synthesis? Q18: The allyl radical has how many Q74: Which set of conditions does not![]()
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents