Indicate which products would be obtained from the chlorination of 1,5-hexadiene at high temperature (500 C) ,using a 1:1 mole ratio of the reactants. 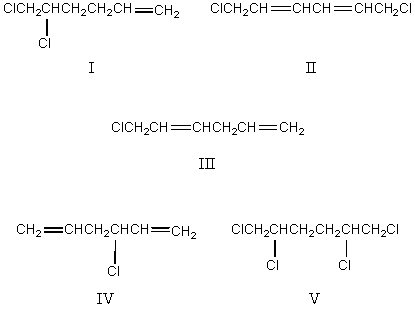
A) I and II
B) II and III
C) III and IV
D) IV and V
E) V and I
Correct Answer:
Verified
Q84: How could the following synthesis be carried
Q85: What is the product of the following
Q86: The accompanying diagram,which describes the fate of
Q88: Which would be the best synthesis of
Q90: The accompanying diagram implies that: 
Q90: Which reagent would convert 1,3-octadiene into 3-octen-2-ol?
A)KMnO4/-OH
B)OsO4
C)H2O2,then
Q91: Which is the only compound which can
Q91: Which of these dienes can undergo the
Q94: Which reaction would produce the following compound?
Q99: A thermodynamically-controlled reaction will yield predominantly:
A)the more/most
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents