Recalling that benzene has a resonance energy of 152 kJ mol-1 and naphthalene has a resonance energy of 255 kJ mol-1,predict the positions which would be occupied by bromine when phenanthrene (below) undergoes addition of Br2. 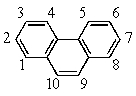
A) 1,2
B) 1,4
C) 3,4
D) 7,8
E) 9,10
Correct Answer:
Verified
Q27: How many different dihydroxybromobenzenes are possible?
A)8
B)7
C)6
D)5
E)4
Q32: We now know that the two Kekule
Q33: Which of the following statements regarding the
Q41: Which of the following would you expect
Q57: Consider the molecular orbital model of benzene.In
Q60: In the molecular orbital model of benzene,how
Q89: Which of the following statements regarding the
Q94: Which of the following statements regarding the
Q99: Which of the following statements regarding the
Q100: The carbon-carbon bonds in benzene are:
A)of equal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents