Amines are known to rapidly undergo pyramidal inversion at ordinary temperatures,preventing the resolution of enantiomeric forms when the amine is a chirality center.The proposed transition state of the inversion process is shown below.Using an orbital diagram,illustrate the hybridization at nitrogen for this proposed transition state. 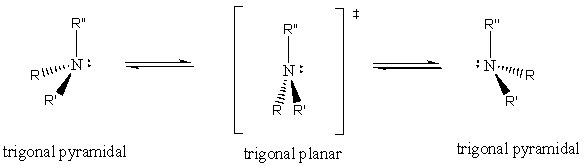
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q120: Which is the major product of the
Q128: Using resonance theory,explain why guanidine is one
Q129: List the following compounds in order of
Q183: Outline the steps involved in the synthesis
Q184: Outline the steps involved in the Gabriel
Q184: Outline the steps involved in the Gabriel
Q188: What final product is likely when cyclohexene
Q193: There are two very versatile synthetic methods
Q194: Arylamines are less basic than alkylamines because
Q199: What final product is likely when cyclohexene
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents