Which reaction is likely to have more products than reactants when the reaction reaches equilibrium?
A) 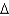 G = -25 kcal/mol
G = -25 kcal/mol
B) 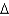 G = -50 kcal/mol
G = -50 kcal/mol
C) 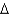 G = -75 kcal/mol
G = -75 kcal/mol
D) 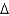 G = -100 kcal/mol
G = -100 kcal/mol
Correct Answer:
Verified
Q4: Which statement best describes the first law
Q7: Suppose that an earthquake hits. Which of
Q9: Which of the following is a closed
Q10: Which of the following best describes a
Q11: Which of the following is a correct
Q14: Which of the following best explains why
Q16: Which of the following is a correct
Q17: Which of the following best describes why
Q18: Which of the following best illustrates the
Q20: Which of the following best describes an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents