Use the following diagram to answer the next problem. 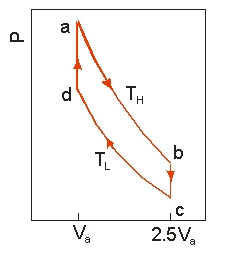 An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
An ideal heat engine uses 0.01 mol of gas and operates between a hot reservoir at
TH = 400 K and cold reservoir at TL = 300 K, in a cycle from a→b→c→d→a. From a→b the gas undergoes an isothermal expansion, changing its volume from Va to 2.5Va. From b→c, the pressure is reduced at a constant volume. From c→d, the gas undergoes an isothermal compression, and from d→a, the pressure is increased at a constant volume until the gas is back at the original condition at a.
-How much heat is absorbed in going from a b?
A) 30.5 J
B) 7.62 J
C) 22.9 J
D) 8.31 J
E) 0.917 J
Correct Answer:
Verified
Q10: Use the following diagram to answer the
Q11: The diagram below is a schematic of
Q12: A refrigerator has a coefficient of performance
Q13: Use the following diagram to answer the
Q14: A heat engine operating between the temperatures
Q16: A heat engine absorbs 64 kcal of
Q17: A heat engine absorbs 150 J of
Q18: If you run a refrigerator in a
Q19: A refrigerator extracts 25 kJ from a
Q20: Two refrigerators,one with a COP of 4.0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents