The Equation Derived by Bohr for the Wavelengths Of Of the Lines in Hydrogen- Like Spectra Is the Of the Lines
The equation derived by Bohr for the wavelengths of of the lines in hydrogen- like spectra is 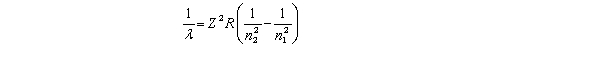 The first member of the Balmer series of hydrogen has = 660 nm.Doubly ionized
The first member of the Balmer series of hydrogen has = 660 nm.Doubly ionized 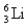 is hydrogen-like.The wavelength of the first member of the Balmer series for doubly ionized
is hydrogen-like.The wavelength of the first member of the Balmer series for doubly ionized 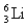 is
is
A) 73 nm
B) 5.9 103 nm
C) 150 nm
D) 60 nm
E) 1.8 10-3 nm
Correct Answer:
Verified
Q16: The radius of the n = 1
Q17: According to the Bohr theory,a hydrogen atom
A)does
Q18: The first Bohr radius,r0,is 0.0529 nm and
Q19: If the potential energy of an electron
Q20: The wavelength of the visible line in
Q22: The radii of the Bohr orbits in
Q23: The energy of the nth level
Q24: What is the energy difference between the
Q25: In the Bohr Model of the hydrogen
Q26: A photon of wavelength 80 nm is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents