According to the Bohr theory,the allowed energy states for the hydrogen atom are given by the relation 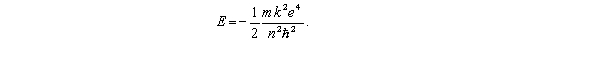 This formula can be readily extended to other hydrogenic (one-electron) systems.The energy of the second level (n = 2) for the doubly ionized lithium atom is
This formula can be readily extended to other hydrogenic (one-electron) systems.The energy of the second level (n = 2) for the doubly ionized lithium atom is
A) -54.4 eV
B) 13.6 eV
C) -30.6 eV
D) -3.4 eV
E) -1.5 eV
Correct Answer:
Verified
Q25: In the Bohr Model of the hydrogen
Q26: A photon of wavelength 80 nm is
Q27: Using Bohr's model,the speed of an
Q28: What is the magnitude of the change
Q29: The red line in the hydrogen emission
Q31: According to Bohr's model,the radius of an
Q32: Light of wavelength 411 nm is observed
Q33: What is the energy difference between the
Q34: An electron in a hydrogen atom jumps
Q35: An electron undergoes a transition from n
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents