For the hydrogen atom in the state n = 2,l = 0,m = 0,the radial probability density is 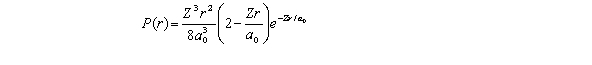 The probability of finding the electron in the range r = 0.04a0 at r = 2a0 is
The probability of finding the electron in the range r = 0.04a0 at r = 2a0 is
A) 0.0167
B) 0.149
C) 0.128
D) 0.0241
E) zero
Correct Answer:
Verified
Q74: For the hydrogen atom in the ground
Q75: The total angular momentum of a hydrogen
Q76: It was _ who showed that the
Q77: The total number of distinct electron states
Q78: Which of the following transitions are allowed
Q80: A compact disc of a CD
Q81: The electron configuration of neon (Z =
Q82: The wavelength of the photon emitted when
Q83: The number of electrons in the M
Q84: A gas-discharge tube filled with hydrogen gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents