For the hydrogen atom in the ground state,the wave function is 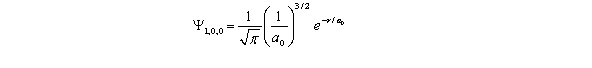 The probability of finding the electron from r = 0 to r = 2a0 is approximately Note:
The probability of finding the electron from r = 0 to r = 2a0 is approximately Note: 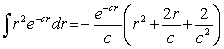
A) 6.34%
B) 12.7%
C) 18.0%
D) 32.3%
E) 76.2%
Correct Answer:
Verified
Q62: The energy of a hydrogen atom in
Q63: An electron has a wave function
Q64: For the hydrogen atom in the
Q65: For the hydrogen atom in the
Q66: For the hydrogen atom in the
Q68: For the hydrogen atom in the
Q69: The total angular momentum is 
Q70: The hydrogen-like elements (valence +1)exhibit a splitting
Q71: For the hydrogen atom in the
Q72: You will never find an electron in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents