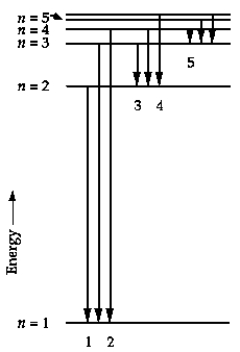
 In the energy-level diagram, the line that corresponds to the longest wavelength in the Balmer series is
In the energy-level diagram, the line that corresponds to the longest wavelength in the Balmer series is
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer:
Verified
Q1: When a gold foil is bombarded with
Q2: J.J.Thomson's model of an atom
A)had electrons embedded
Q3: The first Bohr radius,r0,is 0.0529 nm and
Q15: The radius of the n = 1
Q16: The radius of the n = 1
Q23: The radii of the Bohr orbits in
Q26: A photon of wavelength 80 nm is
Q27: Using Bohr's model,the speed of an
Q37: Bohr's quantum condition on electron orbits
Q39: What is the difference in wavelength between
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents