Multiple Choice
For the hydrogen atom in the state n = 2, l = 0, m = 0, the radial probability density is 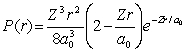 The probability of finding the electron in the range r = 0.04a0 at r = 2a0 is
The probability of finding the electron in the range r = 0.04a0 at r = 2a0 is
A) 0.0167
B) 0.149
C) 0.128
D) 0.0241
E) zero
Correct Answer:
Verified
Related Questions

