 Figure 1
Figure 1
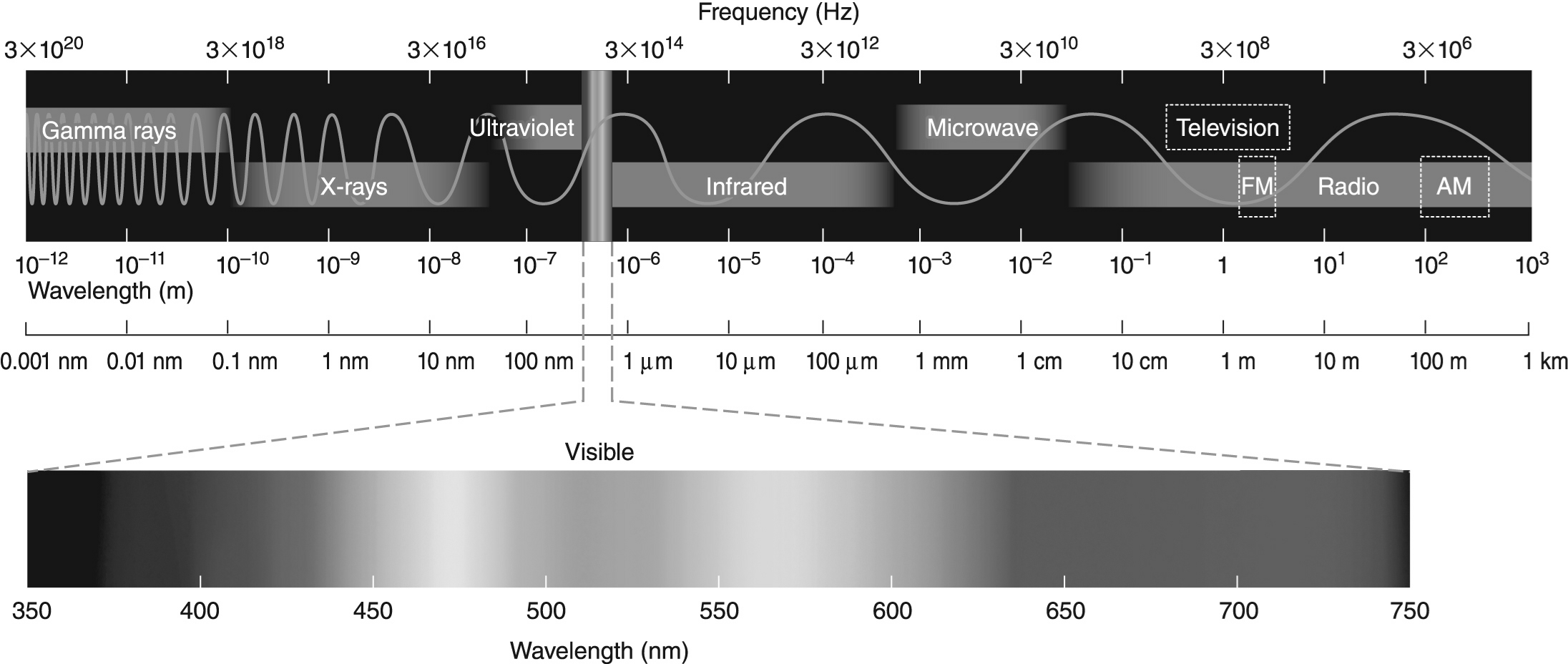
-The difference in energy between the n = 2 and n = 1 electronic energy levels in the hydrogen atom is 1.6 * 10-18 J. If an electron moves from the n = 1 level to the n = 2 level, will a photon be emitted or absorbed? What will its energy be, and what type of electromagnetic radiation is it? Use the electromagnetic spectrum shown above to answer this question.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q64: An asteroid with an albedo of 0.1
Q67: Which of these planets would be expected
Q68: Which of the following factors does NOT
Q70: Imagine you observed three different stars: a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents