You fill two containers with identical amounts of reactants A and B and enzymes 1-4. In the reactions shown below, if product D inhibits enzyme 2 and product F is an allosteric stimulator of enzyme 1, what will be the final result if you add extra product D to the second container? (Assume that both containers are given enough time for the reactions to go to completion.) 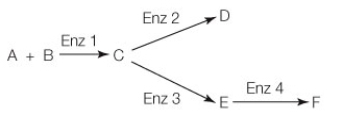
A) The concentration of product C will increase and there will be no change in product F concentration compared to the first container.
B) The concentration of reactants A and B will increase relative to the first container.
C) The concentration of product F will increase in the second container because more of D is converted back to C.
D) The concentration of products E and F will both increase in the second container, since D inhibits enzyme 2.
E) The concentration of product F will increase relative to the first container, since enzyme 2 will have been inhibited from converting as much of C into D.
Correct Answer:
Verified
Q128: You are studying a new species never
Q129: A reaction that has a negative
Q130: You decide to purchase a new water
Q131: Use a graph to explain how temperature
Q132: Amylase is a digestive enzyme that breaks
Q134: Enzymes alter the
A)
Q135: Why is the statement, "ATP produces energy
Q136: You are given an unlabeled enzyme and
Q137: Explain how substrate concentration affects the rate
Q138: The ultimate goal of metabolism is to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents