If an average hydrogen atom in Earth's atmosphere has a velocity of 2.5 km/s,what would be the average velocity of an 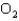 molecule in the Earth's atmosphere? Note that the atomic mass of an oxygen atom is 16 times that of a hydrogen atom.
molecule in the Earth's atmosphere? Note that the atomic mass of an oxygen atom is 16 times that of a hydrogen atom.
A) 0.16 km/s
B) 2.5 km/s
C) 0.62 km/s
D) 0.44 km/s
Correct Answer:
Verified
Q30: If it were not for the greenhouse
Q31: If plant life were to die on
Q32: If you found absorption arising from _
Q33: According to the Ideal Gas law,if you
Q34: Which molecule moves with the fastest average
Q36: If water vapor was released from Venus'
Q37: Based solely on mass,which of the following
Q38: All weather and wind on Earth are
Q39: The main greenhouse gases in the atmosphere
Q40: Earth has roughly _ times more atmospheric
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents