The 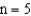 electronic energy level in a hydrogen atom is
electronic energy level in a hydrogen atom is 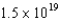 J higher than the
J higher than the 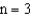 level.If an electron moves from the
level.If an electron moves from the 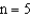 level to the
level to the 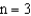 level then a photon of wavelength
level then a photon of wavelength
A) 1.3 nm,which is in the ultraviolet region,is emitted.
B) 1.3 nm,which is in the ultraviolet region,is absorbed.
C) 1,300 nm,which is in the infrared region,is absorbed.
D) 1,300 nm,which is in the infrared region,is emitted
Correct Answer:
Verified
Q32: The light-year is a unit of
A) time.
B)
Q33: A red photon has a frequency of
Q34: Einstein showed that the _ could be
Q35: Which of the following photons carry the
Q36: A nebula emits
A) an absorption spectrum.
B) a
Q38: Which of the following lists different types
Q39: How does the speed of light traveling
Q40: Light with a wavelength of 600 nm
Q42: The average red giant in the night
Q71: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents