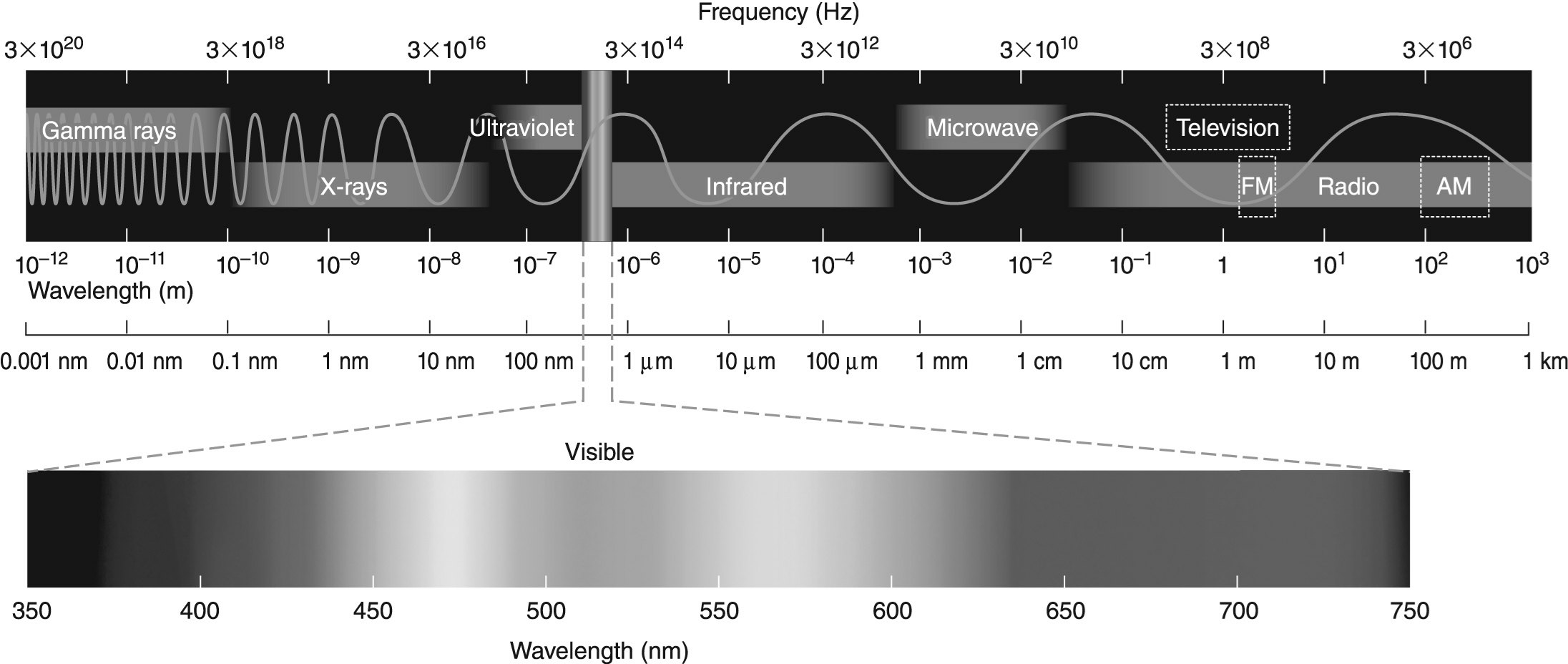
-The difference in energy between the n = 2 and n = 1 electronic energy levels in the hydrogen atom is 1.6 × 10−18 J.If an electron moves from the n = 1 level to the n = 2 level,will a photon be emitted or absorbed? What will its energy be,and what type of electromagnetic radiation is it? Use the electromagnetic spectrum shown above to answer this question.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q68: Which of the following factors does NOT
Q73: If Saturn has a semimajor axis of
Q74: Compare and contrast the wavelengths, frequencies, speeds,
Q78: How are atoms excited and why do
Q82: Astronomers have now found a large number
Q85: If you want a blackbody's peak wavelength
Q89: For a star that lies in the
Q92: Suppose you observe a star emitting a
Q97: If you were driving down a deserted
Q99: Name four physical properties of an object
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents