The thermodynamic parameters at 298 K for the following reaction are given below. 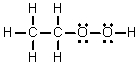
Hº = -64.9 kJ mol-1 Which of the following statements is true of the reaction?
A) Both Hº and Sº favor product formation.
B) Neither Hº nor Sº favors product formation.
C) The entropy term is unfavorable but the formation of ethyl chloride is favored.
D) The entropy term is favorable but the formation of ethyl chloride is not favored.
E) The sign of Gº indicates that the reaction cannot occur as written.
Correct Answer:
Verified
Q1: Addition of hydrogen chloride to the following
Q3: What is the major product for the
Q4: What is the major product for the
Q5: What is the major product for the
Q6: What are the major product(s)formed by treating
Q7: What are possible products for the following
Q8: What is the major product of the
Q9: Treating 1-methylcyclohexene with HCl would yield primarily
Q10: How many compounds are possible from the
Q11: What would be the major product of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents