In which structure(s) below does the oxygen have a formal charge of +1? 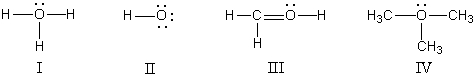 s
s
A) I only
B) II only
C) I and III
D) I and IV
E) I,III,and IV
Correct Answer:
Verified
Q7: Q8: Expansion of the valence shell to accommodate Q9: Which is NOT a correct Lewis structure? Q10: Which of these is a correct electron-dot Q11: Which of the following best describes the Q13: What is the formal charge on oxygen Q14: Which type of bonding is present in Q15: Which of the following compounds contain a Q16: Select the least electronegative element from the Q17: Which structure(s)contain(s)an oxygen that bears a formal![]()
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents